
手機(jī)掃碼訪問(wèn)本站

微信咨詢
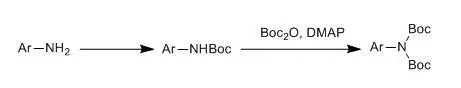
游離氨基在用NaOH 或NaHCO3 控制的堿性條件下用二氧六環(huán)和水的混合溶劑中很容易同Boc2O反應(yīng)得到N-叔丁氧羰基氨基化合物。這是引入Boc常用方法之一,它的優(yōu)點(diǎn)是其副產(chǎn)物無(wú)多大干擾并容易除去。有時(shí)對(duì)一些親核性較大的胺,一般可在甲醇中和Boc酸酐直接反應(yīng)即可,無(wú)須其他的堿,其處理也方便。
反應(yīng)實(shí)例
一、氨基酸Boc保護(hù)示例
Oskar Keller,Walter E. Keller, Gert van Look et al., Org. Syn., 63, 160
A 4-L, four-necked, round-bottomed flask, equipped with anefficient stirrer, a droppingfunnel, reflux condenser, and thermometer is charged with a solution of 44 g (1.1 mol) of sodium hydroxidein 1.1 L of water. Stirring isinitiated and 165.2 g (1 mol) of L-phenylalanine is added at ambienttemperature, and then diluted with 750 mL of tert-butyl alcohol. To the well-stirred, clearsolution is added dropwise within 1 hr, 223 g(1 mol) of di-tert-butyl dicarbonate.A white precipitate appears during addition of the di-tert-butyldicarbonate. After a short induction period, the temperature rises toabout 30–35°C. Thereaction is brought to completion by further stirring overnight at roomtemperature. At this time, the clear solution will have reached a pH of7.5–8.5. The reaction mixture is extracted two times with 250mL of pentane, and the organic phase isextracted three times with 100 mL of saturated aqueous sodium bicarbonate solution. The combined aqueous layersare acidified to pH 1–1.5 by careful addition of a solution of 224 g (1.65 mol) of potassium hydrogensulfate in 1.5 L ofwater. The acidification is accompanied by copious evolution of carbon dioxide. The turbid reaction mixture is thenextracted with four 400-mL portions of ethyl ether. The combined organic layers are washed twotimes with 200 mL of water, dried over anhydrous sodiumsulfate or magnesium sulfate, and filtered.The solvent is removed under reduced pressure using a rotaryevaporator at a bath temperature not exceeding 30°C. The yellowish oil that remains istreated with 150 mL of hexaneand allowed to stand overnight. Within 1 day the following portions of hexane are added with stirring tothe partially crystallized product: 2 × 50 mL, 4 × 100 mL, and 1 × 200 mL.The solution is placed in a refrigeratorovernight; the white precipitate is collected on a Büchnerfunnel and washed with cold pentane. Thesolid is dried under reduced pressure at ambient temperature to constant weightto give a first crop. The mother liquor is evaporated to dryness leaving ayellowish oil, which is treated in the same manner as described above, giving asecond crop. The total yield of pure white N-tert-butoxycarbonyl-L-phenylalanine is 207–230 g(78–87%), mp 86–88°C, [α]D20 + 25.5° (ethanol c 1.0).
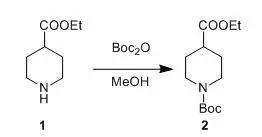
二、 Boc酸酐在甲醇中與胺直接反應(yīng)
Boc2O (262 g, 1.2 mol) in MeOH (250 ml) was added toa soluton of compound 1 (157.2 g,1.0 mol) in MeOH (350 ml) at 10°C,and the resulting mixture was stirred at room temperature for 2 h. N1,N1-dimethylethane-1,2-diamine (26 g, 0.3 mol) was added and the mixture was stirredat room temperature for 15 min. The solvent was removed in vacuo, and theresidue was dissolved with ethyl acetate (750 ml). The combined organics werewashed with 1 N HCl (2 x 250 ml) and brine (2 x 250 ml), dried over sodiumsulfate and filtered. The solvent was removed to give compound 2 (250 g, 96%), which was used directly in thenext step.
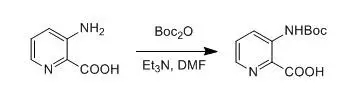
三、芳胺的單Boc保護(hù)示例
Luo, Qun-Li;Liu, Zhi-Ying et al., J. Med. Chem., 2003, 46(13), 2631-2640
3-Aminopyridine-2-carboxylic acid(5.02 g, 36 mmol) was suspendedin 60 mL of dry DMF, and Et3N (15.2 mL, 108 mmol) was added dropwise at roomtemperature. To the resulting brown solution was added Boc2O (11.80 g, 54 mmol). After being stirred for 10min, the mixture was heated at 40-50 °Covernight. The reaction mixture was poured into water and was then extractedwith EtOAc (2 X 50 mL). The aqueous phase was acidified to pH 4-5 with 2 M aqueous HCl and then extracted with CH2Cl2(3 X 50 mL). The combined organic phases were thenprocessed in the usual way and chromatographed (13:1 CHCl3/MeOH) to yieldthe desired product (4.2 g,49%).
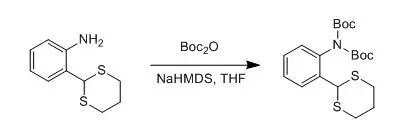
四、 芳胺的雙Boc保護(hù)示例
Macleod, Calim;Mckieman, Gordon J et al., J. Org. Chem., 2003, 68(2),387-401
A solution of NaHMDS (22.0 mL, 22.0 mmol, 1 M in THF) was added to a solution of the amine (2.11 g, 10.0 mmol) and (Boc)2O (5.46 g, 25.0 mmol) in THF (50 mL) at 0°C under nitrogen. The reaction was allowedto warm to rt and stirred for 16 h. After this time, the reaction was poured intowater, extracted into CH2Cl2(2 X 25 mL),washed with water (2 X 25 mL), dried over Na2SO4, and concentrated to yield a white-yellowsolid. Recrystalization from petroleum ether (40-60 °C) gave the imide as needles (3.21 g, 7.80 mmol, 78%). Rf (hexane/ CH2Cl2 1:9, SiO2): 0.10. Mp:106-109 °C.
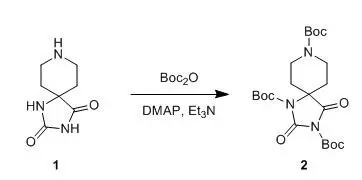
五、 酰胺的Boc保護(hù)示例
Lars G. J. Hammarström, Yanwen Fu et al., Org. Syn., 81, 213
A 2000-mL, three-necked, round-bottomed flask equippedwith an argon inlet adapter, glass stopper, and an overheadmechanical stirrer is charged with a suspension of the hydantoin 1 (26.0 g,154 mmol) in 1000 mL of 1,2-dimethoxyethane. Triethylamine (15.7 g, 154mmol) is added in one portion, and the resulting white suspension isstirred for 30 min. Di-tert-butyl dicarbonate (168.0 g, 770mmol) is then added by pipette, followed by4-dimethylaminopyridine (DMAP)(0.2 g, 1.5 mmol). Six additional 0.2 g-portionsof DMAP are added at 12 hr intervals during the course of the reaction.The reaction mixture is stirred vigorously for a total of 72 hr, and the resultinglight yellow solid is then collected in a Büchner funnelusing suction filtration. The filtrate is concentrated to a volume of 60 mL byrotary evaporation, and the resulting solution is cooled to 15°C. The precipitate which appears iscollected using suction filtration, added to the first crop, and the combinedsolids are dissolved in 500 mL of chloroform. This solution is washed with three 200-mL portions of 1.0N HCl, and the combinedaqueous phases are extracted with 100 mL of chloroform. The combined organic layers are washed with 100 mL of saturated aq NaHCO3solution and 100 mL of brine,dried over anhydrous MgSO4,filtered, and concentrated by rotary evaporation. The resulting solid is driedat room temperature at 0.01 mmfor 24 hr. The resulting finely ground light yellow solid is suspended in 400 mL of diethyl ether in a 1000-mL, round-bottomed flask equipped with a magnetic stirbar, stirred for 2 hr, and filtered on aBüchner funnel washing with four 50-mL portions of diethyl ether. The product is dried under vacuum (85°C; 0.5 mm) for 24 hr to give 60.0–65.3 g (83-90%)of 2 as a ivory-colored solid.
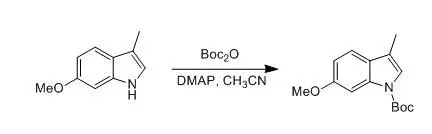
六、吲哚Boc保護(hù)示例
G. Tong; P. Ruiyan et al., J. Org.Chem., 1997, 26, 9298
To a solution of6-methoxy-3-methylindole (5.0 g,31 mmol) in distilled acetonitrile (150 mL) were added di-tertbutyl dicarbonate(7.44 g, 34.1 mmol) andDMAP (0.195 g, 1.6 mmol).The reaction mixture was stirred at rt for 12 h. The solvent was removed underreduced pressure. The residue was dissolved in CH2Cl2(100 mL) and washed with an aqueous solution of 1 N HCl (2 x 50 mL). Theaqueous layer was extracted with CH2Cl2(3 x 30 mL). The combined organic ayers were dried (K2CO3). Afterremoval of solvent under reduced pressure, the residue was solidified to affordthe product (8.12 g, 99%) as a yellow solid: mp 45-46 °C.
本文非原創(chuàng)內(nèi)容,版權(quán)歸原作者所有